Medtronic deal with Aetna ties insulin pump payment to patient results
This does not feel right...
My opinion.... Medtronic has way too much power in the insulin pump space. You may also want to check out Mike Hoskin's thoughts on the subject over at Diabetes Mine.
By Bill Berkrot Reuters
Medtronic Plc said on Monday it signed an agreement with health insurer Aetna Inc under which payment for its insulin pump systems will be tied to how well diabetes patients fare after switching from multiple daily insulin injections.
The deal is the latest example of the move toward contracts for prescription drugs and medical devices that attempt to bring down soaring healthcare costs by tying reimbursements to whether the products achieve their intended results.
The deal with Aetna will measure health outcomes for patients who transition to one of three Medtronic pumps that self-adjust to keep blood sugar levels in proper range based on patients' individual needs for insulin.
"This agreement reinforces our shift towards value-based healthcare," Hooman Hakami, president of the Medtronic diabetes group, said in a statement. "We know technology alone isn't enough and ultimately improved outcomes are what matter."
Patients with type 1 diabetes and those with type 2 who have progressed to the need for insulin typically check blood sugar levels several times a day and inject insulin as needed. The pumps eliminate that chore.
Medtronic declined to discuss financial details of the Aetna agreement, but said such deals tie revenue to achievement of clinical improvement targets, as well as shared savings for delivering on or exceeding clinical outcomes and cost targets.
Suzanne Winter, vice president of the Medtronic diabetes group in the Americas, said the Aetna agreement will initially focus on whether patients on its pumps achieve their A1c targets, a commonly used measure of blood sugar levels. The American Diabetes Association recommends A1c levels below 7.
In the future it may look at other measures, such as hypoglycemia episodes, time in proper glycemic range, and patient satisfaction, Winter said.
Medtronic already has an agreement with UnitedHealth Group Inc that is moving toward including patient outcomes and other metrics, such as total cost of care, and the company is discussing similar deals with other insurers, Winter said.
Pharmaceutical companies are also beginning to embrace reimbursement options that take patient outcomes into consideration.
U.S. biotech Amgen Inc, in an effort to improve patient access to its expensive new cholesterol drug Repatha, has offered contract options that include refunding the cost of the drug if patients suffer a heart attack or stroke while on the medicine intended to prevent them.
(Reporting by Bill Berkrot; Editing by David Gregorio)
Dexcom Announces FDA Approval of G5 Mobile App for Android Devices
SAN DIEGO--(BUSINESS WIRE)-- DexCom, Inc. (Nasdaq: DXCM) the leader in continuous glucose monitoring (CGM) for people with diabetes, is pleased to announce the Food and Drug Administration (FDA) approval of the Dexcom G5 mobile app for Android devices. Beginning in June, Android users will have access to the free app for the Dexcom G5 Mobile CGM System, allowing people with diabetes to view and monitor their glucose levels on their mobile devices to manage their diabetes in real time. The Dexcom G5 Mobile CGM System is the first and only CGM platform available for Android in the United States, complementing the 2015 iOS launch.
Download for free at the Google Play store here: bit.ly/AndroidDexcomG5MobileApp
The Dexcom G5 Mobile is a compact CGM system that works to display real-time glucose activity on certain approved display devices. The launch of Dexcom G5 Mobile for Android allows people to manage their diabetes in a more personal and discrete way by providing glucose data on their Android mobile device, as well as the ability to share it safely and conveniently. This empowers them to make informed and timely decisions about their diabetes, resulting in better health outcomes.
"Providing Android users with access to the Dexcom G5 Mobile CGM System has been a priority for Dexcom," said Kevin Sayer, President and CEO, Dexcom. "The new Android app has been thoughtfully designed with customer needs and feedback in mind. It focuses on delivering technology that empowers users by putting critical glucose information on their phones and is compatible with the most popular Android devices currently in the market."
Once commercially available, the new app will make the Dexcom G5 Mobile available on millions of additional phones in the United States. The Dexcom G5 Mobile app for Android will initially be available on several Android devices from Samsung, Motorola and LG, as well as Android Wear watches.
A current list of compatible devices can be found at www.dexcom.com/compatibility.
The Juicebox Podcast is featured on iTunes!
A huge thank you to Apple Podcasts and iTunes for shining a spotlight on episode #111 of the Juicebox Podcast.
Guest: Actor from 'Baby Daddy' and life-long type 1, Derek Theler...
You can listen to this and past episodes on Apple Podcast. Subscribe today!
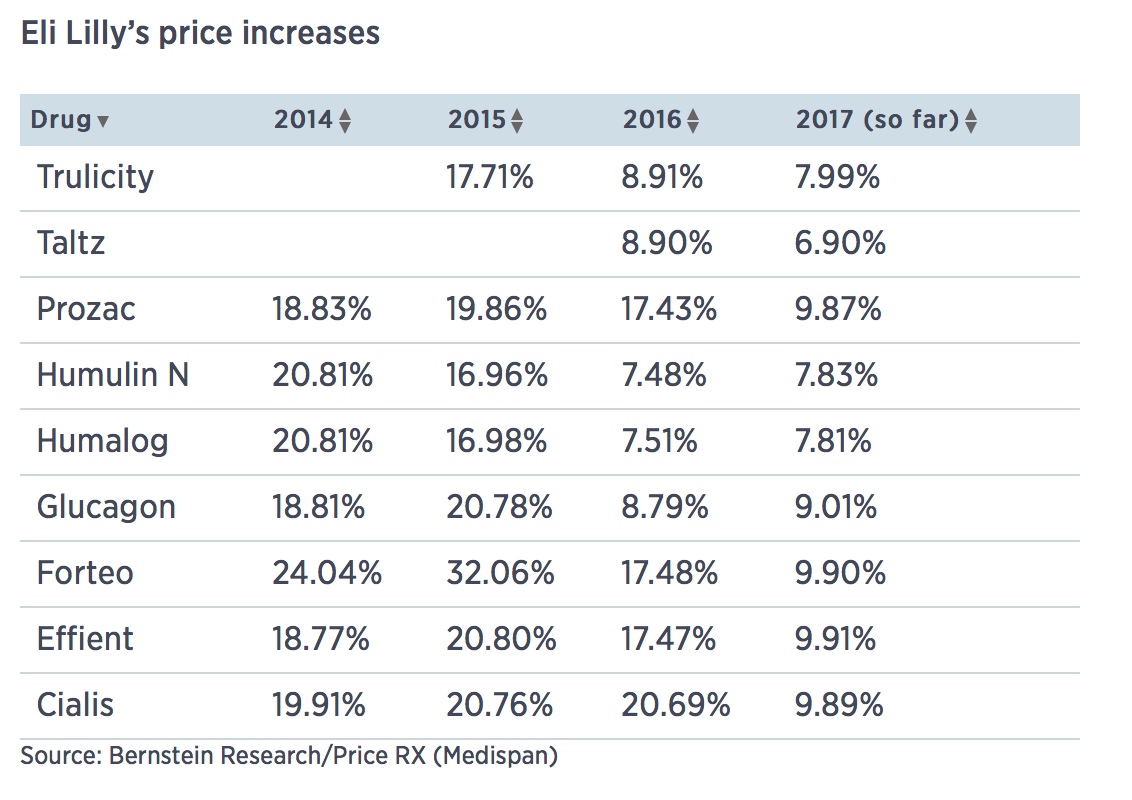
Eli Lilly raised prices on 9 drugs last week
Humalog and Humulin (7.8 percent)
Indianapolis drug giant Eli Lilly raised list prices of nine of its medicines last week between 6 and 10 percent, according to data obtained by CNBC.
The increases, taken on May 2, were for drugs including the blood thinner Effient (9.9 percent), the psoriasis drug Taltz (6.9 percent) and the insulins Humalog and Humulin (7.8 percent).
The increases fit a pattern at Lilly and many other drugmakers of single-digit percentage hikes once or twice a year, despite political pressure and intense scrutiny of the practices. Lilly has come under fire for the price of its insulin drugs in particular, leading Senator Bernie Sanders to call for a federal investigation into collusion. Lilly and other insulin makers have denied any such activity.
Lilly confirmed the price increases in an emailed statement to CNBC, and noted the list prices "do not reflect the significant discounts and rebates that we provide to ensure patients have adequate access to our medicines."
"The net price increase that Lilly recognizes is significantly less," spokesman Mark Taylor wrote. "In fact, in 2016, the average discount to list price on our U.S. portfolio rose to 50 percent and net prices rose just 2.4 percent in the U.S."
You can read the entire article on CNBC
Medicare Announces Criteria Covering Dexcom G5 Mobile CGM for All People with Diabetes
March 24, 2017
Medicare Announces Criteria Covering Dexcom G5 Mobile CGM for All People with Diabetes on Intensive Insulin Therapy
SAN DIEGO--(BUSINESS WIRE)-- DexCom, Inc. (NASDAQ:DXCM), the leader in continuous glucose monitoring (CGM) for people with diabetes, is pleased to announce that the U.S. Centers for Medicare & Medicaid Services (CMS) has published an article clarifying criteria for coverage and coding of the Dexcom G5 Mobile system, the only therapeutic CGM under this CMS classification. People covered by Medicarewho have either Type 1 or Type 2 diabetes and intensively manage their insulin will now be able to obtain reimbursement.
"This is a new era and a huge win for people with diabetes on Medicare who can benefit from therapeutic CGM," said Kevin Sayer, President and Chief Executive Officer, Dexcom. "This decision supports the emerging consensus that CGM is the standard of care for any patient on intensive insulin therapy, regardless of age."
According to CMS, therapeutic CGM may be covered by Medicare when all of the following criteria are met:
- The beneficiary has diabetes mellitus; and,
- The beneficiary has been using a home blood glucose monitor (BGM) and performing frequent (four or more times a day) BGM testing; and,
- The beneficiary is insulin-treated with multiple daily injections (MDI) of insulin or a continuous subcutaneous insulin infusion (CSII) pump; and,
- The patient's insulin treatment regimen requires frequent adjustment by the beneficiary on the basis of therapeutic CGM testing results.
In order to be included in this category, the system must be defined as therapeutic CGM, meaning you can make treatment decisions using the device. Dexcom G5 Mobile is the only system approved by the FDA to meet that criteria.