Human GI Cells Retrained to Produce Insulin
I don't post nearly all of the stories that I read about cure research, but I found this one to be particularly interesting and wanted to pass it on.
I don't post nearly all of the stories that I read about cure research, but I found this one to be particularly interesting and wanted to pass it on.
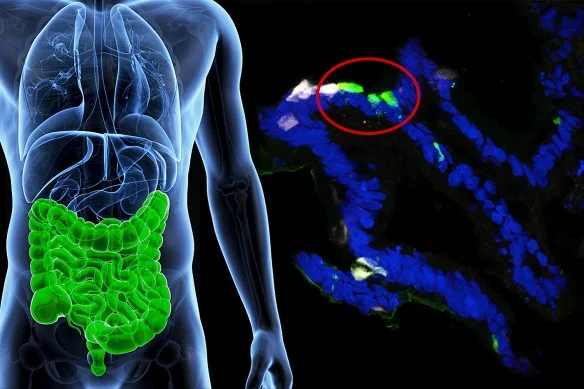
Human gastrointestinal cells from patients were engineered to express insulin (fluorescent green) in the lab. (Image by Columbia University Medical Center.)
Excerpts from a Columbia University Medical Center press release:
By switching off a single gene, scientists at Columbia University’s Naomi Berrie Diabetes Center have converted human gastrointestinal cells into insulin-producing cells, demonstrating in principle that a drug could retrain cells inside a person’s GI tract to produce insulin.
The finding raises the possibility that cells lost in type 1 diabetes may be more easily replaced through the reeducation of existing cells than through the transplantation of new cells created from embryonic or adult stem cells.
After seven days, some of the cells started releasing insulin and, equally important, only in response to glucose.
You can read the entire press release here
FDA Approves Inhalable Diabetes Drug Afrezza
I want to be 100% clear that my next statement is based on absolutely nothing but my gut instinct. I may very well be completely wrong but...
I would never let Arden inhale insulin, it just doesn't sound like a good idea to me.
That said, I hope that I'm wrong and that this helps countless people...
From ABC News/AP News
The Food and Drug Administration on Friday approved a long-delayed inhalable diabetes medication to help patients control their blood sugar levels during meals.
The FDA cleared MannKind Corp.'s drug Afrezza, a fast-acting form of insulin, for adults with the most common form of diabetes that affects more than 25 million Americans. The approval decision comes more than three years after the agency first asked MannKind to run additional clinical studies on the drug.
The rest of this news story can be found here.
The FDA News release is here.
NEW A1c guidelines from the American Diabetes Association
The American Diabetes Association just announced their new position on A1c targets, for children under the age of 19, who live with type I diabetes. What follows are snippets from the ADA story.
The Association now recommends that children under the age of 19 diagnosed with type 1 diabetes strive to maintain an A1C level lower than 7.5 percent. Previously, target blood glucose levels – as measured by the A1C, a test that reflects average blood glucose levels over several months – could be as high as 8.5 percent for children under 6 years of age, 8.0 percent for children 6-12 years of age and 7.5 for adolescents under the Association’s guidelines. These targets were set because of concerns over complications caused by low blood glucose, or hypoglycemia.
“The evidence shows that there is a greater risk of harm from prolonged hyperglycemia that would occur if children maintained an A1C of 8.5 percent over time. This is not to say we are no longer concerned about hypoglycemia, but we now have better tools to monitor for hypoglycemia,” said Jane Chiang, MD, Senior Vice President, Medical and Community Affairs, American Diabetes Association and one of the lead authors on the Association’s Position Statement. “The 7.5 percent target is evidence-based; however, we want to emphasize that blood glucose and A1C targets must be individualized to safely achieve the best outcomes.”
I want to urge you to click over to the ADA now and find out more about the changes and the reasons behind them.
We've been aggressive with Arden's BGs for the past two years and the results have been nothing but fantastic. Arden received her latest A1c test yesterday and I'll be posting a blog tomorrow about her result and how we've been able to achieved it.
NPR: Father Devises A 'Bionic Pancreas' To Help Son With Diabetes
So many times I tell people, "You'd have to live with us for a few days to really understand type I diabetes".
NPR posted a wonderful audio interview with Ed Damiano, the man who is working on the 'Bionic Pancreas' at Boston University. I've embedded Rob Stein's report here for you to listen to... after you're finished I encourage you to head over to NPR's site to read the entire interview.
Image by Ellen Webber for NPR
DexCom’s Forthcoming Mobile App Platform to Integrate Data from Insulet’s OmniPod System
Well, well... This sounds like a positive step!
From Market Watch...
SAN DIEGO, Jun 13, 2014 (BUSINESS WIRE) -- Insulet Corporation and DexCom, Inc. announced today their intention to enable DexCom’s mobile App platform, which is currently under development, to integrate data from Insulet’s OmniPod System. This will be the first version of a mobile App that is capable of incorporating glucose and other diabetes-related data from patients’ devices and displaying the integrated data via a smartphone App. This event also marks the start of DexCom's open architecture approach to diabetes-related data which will include an “approved by DexCom” indication to validate the authenticity of devices and Apps integrating DexCom CGM data.
“Providing OmniPod users and their healthcare providers with easier access to their management data is another key step in making diabetes a smaller part of life,” said Duane DeSisto, President and Chief Executive Officer of Insulet. “Through DexCom’s mobile App platform, OmniPod users will have greater access to the data that is so essential for understanding and improving diabetes management. We are proud to help people with diabetes gain greater and easier control over their disease and we remain dedicated to providing advancements that further this mission.”
“More than ever, we believe that effective diabetes management depends on timely and accurate data. That is why we are excited to complete development of the App and integrate data from Insulet’s OmniPod system,” said Terry Gregg, DexCom’s CEO.
Read the entire announcement here.